Summary
Vanderbilt inventors have developed a one-step hydrosilylation synthesis of azide surfaces for the preparation of click chemistry compatible substrates. In this process, an organic azide is formed in a single step on a hydrogen-terminated silicon support, yielding a surface that is ready to undergo click reactions as desired. Simple, efficient, and versatile, click chemistry is widely used and is particularly useful for biosensing applications. A click reaction can be utilized to attach a molecular or biological probe for point-of-care diagnostics and chemical screening.
Overview
The azide film provides a modifiable surface, and flexible linkage chemistry enables broad sensing and array possibilities using commercially available capture molecules.
Addressed Need
Diagnostic sensors based on click chemistry are versatile tools for rapid, accurate and specific detection of target molecules, metal ions, biomolecules, disease biomarkers, and more. By providing an organo-azide, a flexible platform that serves as a foundation to which alkyne-containing capture/sensing molecules of choice can be attached. The attachment by click reaction requires the presence of an azide functional group on the substrate’s surface. Hydrosilylation chemistry is utilized to attach azide groups to hydrogen-terminated silicon substrates; however, current hydrosilylation methods employ multi-step, slow, and expensive processes. The Vanderbilt technology affords an inexpensive, single-step process to create such azide-attached silicon substrates that is compatible with and easy to adopt in the conventional manufacturing workflow. Vanderbilt views this enabling technology as a beneficial intermediate-step technology useful for a variety of applications.
Technology Description
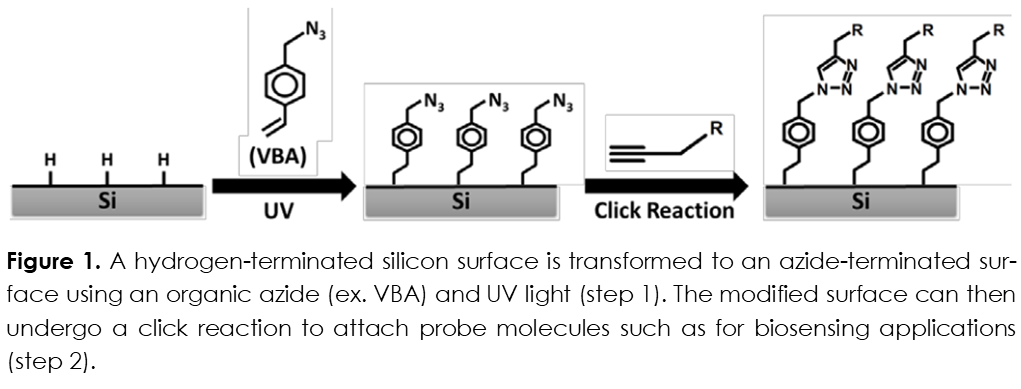
In the present technology, a one-step hydrosilylation reaction is used to attach an organic azide molecule to a hydrogen-terminated silicon surface (including both flat and porous silicon architectures). The organic azide is deposited onto the surface and then irradiated with UV light, which results in the attachment of the azide molecule to the silicon surface. The azide-terminated surface is now suitable for the attachment of molecular or biological probes. These probes provide constituents for constructing both biosensors and screening libraries.
Commercial Applications
The current process can be adopted as an intermediate step in the manufacture of azide-terminated silicon substrates. Such substrates are used for the application of click chemistry in materials science, drug delivery platforms, drug discovery, imaging, and biosensing.
Intellectual Property Status
A patent application has been filed.